Atoms
To comprehend how objects can generate a positive or negative charge, it is necessary first to understand the particles that comprise these objects. Atoms are the basic building blocks of matter and consist of three (3) basic particles: protons, neutrons, and electrons. Of these three, protons and electrons carry a charge, while neutrons, on the other hand, is neutral, which means it has no net charge.
Protons and neutrons reside at the nucleus, which can be found at the very center of the atom, while electrons orbit the nucleus ($\fref{1}$).
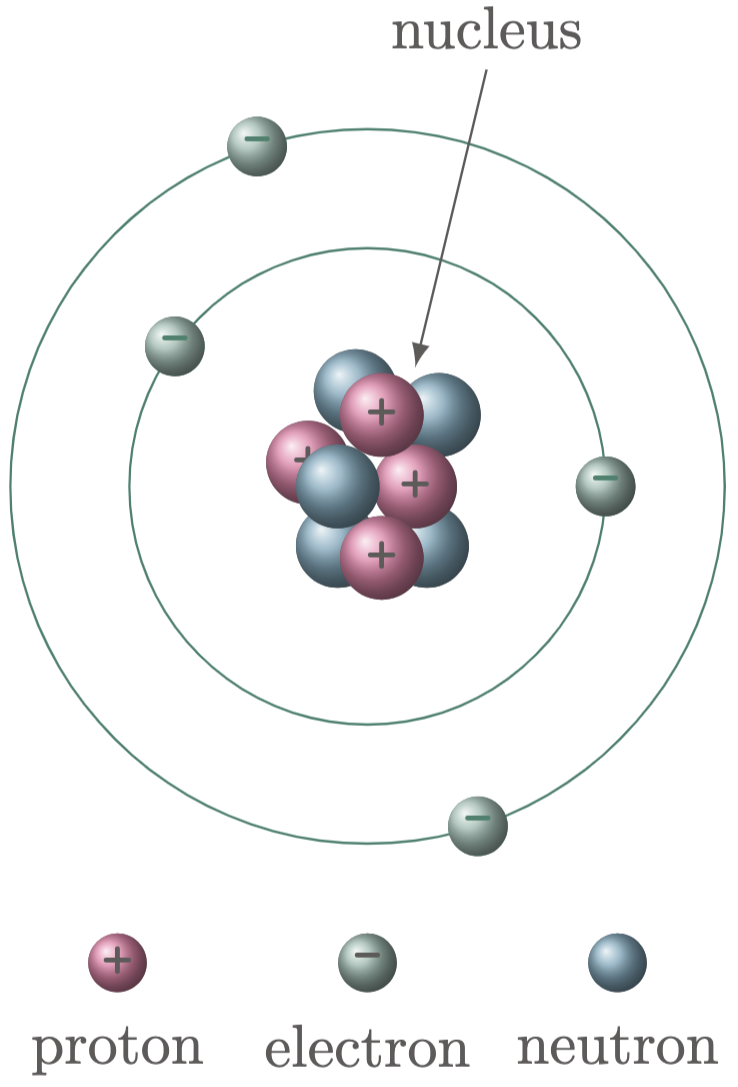
Figure 1: Visual representation of an atom
Electric Charge
A charge is defined as an electrical property of the atomic particles that consists of matter. It is measured in coulombs (C), named after French Engineer and Physicist Charles-Augustin de Coulomb.
The symbol $e$ usually denotes the elementary unit of charge. It is defined in terms of the charge of either a proton or an electron. Since a proton has a net positive charge, we can denote it as $e$. While an electron carries the same magnitude but has an opposite charge as the proton, hence we can represent it as $-e$.
\[\begin{align*} e &= \text{charge of a proton} \\ -e &= \text{charge of an electron} \end{align*}\]In most cases, an atom contains an equal number of protons and electrons, canceling out the charge. The number of protons or electrons in a neutral atom is referred to as the element’s atomic number.
However, when the number of protons and electrons in an atom are not equal, creating a charge, the atom is referred to as an ion.
If there are more protons than electrons, it will result in a net positive charge, which will then be called a cation.
And when there are more electrons than protons, which will result in a net negative charge, it would be called an anion.
The imbalance of numbers of protons and electrons happens when an atom gains or loses electrons, and this phenomenon is called ionization.
Unit of Charge
An electron’s charge ($e$) is quantified as being equal to $1.602 \x 10^{-19}$ coulombs $(\mathrm{C})$ and has a negative charge. What this means is that every electron intrinsically contains $–1.602 \x 10^{-19} \un{C}$, and it requires $6.24 \x 10^{18} \un{electrons}$ to gain one (1) coulomb of charge.
Similarly, a single proton contains $1.602 \x 10^{-19} \un{C}$ in magnitude but positive in charge. Hence, one proton contains $+1.602 \x 10^{-19} \un{C}$.
The presence of equal numbers of protons and electrons in an atom makes it neutrally charged (The algebraic sum of charges is zero).
$$\begin{align} e &= 1.602 \times 10^{-19} \un{C/proton} \\ e &= -1.602 \times 10^{-19} \un{C/electron} \end{align}$$
The mass of an atom is concentrated at the nucleus since the mass of a proton and a neutron is relatively larger compared to the mass of an electron. The mass of each particles are:
\[\begin{align*} \text{mass of electron} &= m_e = 9.109\times 10^{-31}\un{kg}\\ \text{mass of proton} &= m_p = 1.673\times 10^{-27}\un{kg}\\ \text{mass of neutron} &= m_n = 1.675\times 10^{-27}\un{kg}\\ \end{align*}\]
